Learning Outcomes
i. Students will be able to explain the concept of phenols and their relationship to alcohols.
ii. Students will understand the nomenclature of phenols using IUPAC conventions.
iii. Students will be able to describe the structure of phenols, including the resonance stabilization of the hydroxyl group.
iv. Students will explain the increased acidity of phenols compared to alcohols.
Introduction
Phenols, a class of organic compounds, are characterized by the presence of a hydroxyl (-OH) group attached directly to a benzene ring. Unlike alcohols, where the hydroxyl group is attached to an alkyl or aryl group, phenols exhibit unique properties due to the electron-rich nature of the benzene ring.
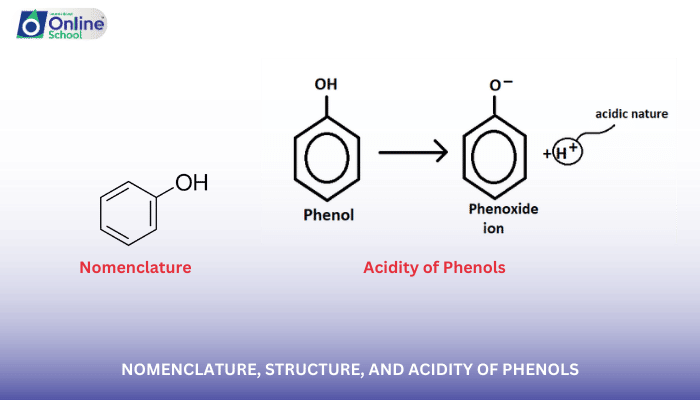
i. Nomenclature of Phenols
The nomenclature of phenols follows the International Union of Pure and Applied Chemistry (IUPAC) conventions for organic compounds.
Common Names: Phenols often have common names based on their sources or properties, such as phenol (carbolic acid), resorcinol (meta-dihydroxybenzene), and catechol (o-dihydroxybenzene).
IUPAC Names: According to IUPAC rules, the hydroxyl group takes precedence over substituents on the benzene ring. The position of the hydroxyl group is indicated by a prefix (ortho-, meta-, or para-) or by numbering the carbon atoms of the benzene ring.
ii. Structure of Phenols
Phenols exhibit a unique resonance stabilization due to the interaction of the lone pair of electrons on the oxygen atom with the pi electron clouds of the benzene ring. This resonance stabilization enhances the stability of the phenol compared to an alcohol and affects its acidity.
iii. Acidity of Phenols
Phenols are more acidic than alcohols due to the electron-withdrawing nature of the benzene ring. The resonance stabilization of the phenoxide ion, the conjugate base of phenol, is more effective than that of the alkoxide ion, the conjugate base of an alcohol. This increased stability of the phenoxide ion makes phenols more likely to dissociate and release protons, leading to their higher acidity.
Phenols, with their unique structure and properties, play a significant role in various fields. Understanding the nomenclature, structure, and acidity of phenols is essential for appreciating their diverse applications in pharmaceuticals, plastics, and the production of synthetic dyes. Phenols continue to be an active area of research, with potential for further discoveries and technological advancements.